-
The 15th APAC Conference will be held on Tuesday, April 21, 2026, in Tokyo, Japan.
-
The new president message from JPMA
-
The 14th APAC Meeting : Post-Meeting Follow-Up (Presentation Materials Available)
-
APAC welcomes a new member
APAC is delighted to welcome Pharma Group (PG) as a new member.
Click Here For More Info about Pharma Group! -
The 14th APAC Meeting Post-Ups: Date and Venue
Save the date to join us at the 14th APAC Meeting in Tokyo, April 22, 2025.
The Meeting will be held at Keidanren Kaikan located in 1-3-2, Otemachi, Chiyoda-ku, Tokyo. -
APAC e-labeling EWG is excited to announce an upcoming virtual event,
“ePI is on FHIR Around the Globe,” co-hosted with Gravitate Health.Date: 21st October, 2024
Place: Online
Participation fee: free
Please see below for details.AGENDA & TOPICS
-
Introduction to key ePI developments around the globe.
-
EU Perspective: Lessons learned from the European Medicines Agency (EMA) ePI pilot.
-
Middle East: A demo of the Jordan ePI system/app.
-
Asia Pacific: Taiwan’s regulator-led ePI initiatives and future plans.
-
Norway: Felleskatalogen’s perspective on the potential of ePI in healthcare.
Panel Discussion & Q&A
The event will conclude with a panel discussion, where attendees can engage with the speakers and ask questions about ePI’s potential in transforming global healthcare systems.WHY ATTEND?
This event offers a unique opportunity to gain insights into how FHIR ePI is creating a unified international standard, facilitating better access to medicinal product information, and enabling improvements in patient care and safety. The discussions will also address the benefits and challenges of adopting ePI in different regions.
This session is open to all stakeholders across healthcare and regulatory sectors, and it promises to be the first in a series of similar global discussions aimed at fostering collaboration on ePI innovations.Registration site is HERE!
-
-
The 13th Asia Partnership Conference of Pharmaceutical Associations (APAC)
Theme: “We reaffirm the APAC’s mission and fulfill it for patients in Asia”The 13th APAC conference was held on Tuesday, April 23, 2024 at KeidanrenKaikan, Tokyo with 231 participants out of 13 economies at the venue and almost 400 online audiences from all over Asia.
The conference began with a congratulatory speech by Dr. David Reddy, the new Director General of IFPMA, and a keynote speech by PMDA Chief Executive Dr. Yasuhito Fujiwara. As in last year, five sessions were held, and many speakers were selected from regulatory authorities in each economy, who engaged in lively discussions.
Sessions held are Regulations and Approvals, Drug Discovery Alliances, e-labeling, Manufacturing-Quality Control-Supply, and Asian UHC.
After the session, LDP Upper House Member Keizo Takemi, who was nominated Minister of Health, Labor and Welfare in September 2023, came to the venue and shared with us the international initiatives that the Japanese government will be focusing on in the future.
Parliamentary Vice-Minister Akihisa Shiozaki also attended the reception and gave a wonderful congratulatory speech.
The 14th conference is scheduled to be held in Tokyo in April 2025, so we look forward to your continued support and advice.
Please check the presentation materials at each session, and the group photo, which is posted on a special website until the end of June. -
The 12th APAC Meeting : Post-Meeting Follow-Up (Presentation Materials Available)
-
The 12th APAC: Register Now!
The 12th APAC will be held in Tokyo, 18 April, 2023. The online registration system is now open.
For more information, please follow this link.
-
The renewed president message for the FY 2022
-
The 11th APAC Meeting : Post-Meeting Follow-Up (Presentation Materials Available)
-
The 11th APAC will be held on April 5th, 2022
-
The new president message from JPMA
Please visit ABOUT US – Welcome message. You will find a message from Mr. Yasushi Okada, the new president of JPMA elected on May 20th, 2021.
-
The 10th APAC Meeting : Post-Meeting Follow-Up (Presentation Materials Available)
-
10th APAC Post Ups : Program book
-
The 10th APAC Conference Post-Ups: Meeting Information
-
The 10th APAC will be held April 13, 2021
-
General Assembly unanimously approved Nakayama George to retain his presidency 1 more year.
-
The 9th APAC Meeting: Post-Meeting Follow-Up (Activity Summary)
-
JPMA appoints new leadership to the Secretariat
On March 23, The Japan Pharmaceutical Manufacturers Association (JPMA) named Junichi Shiraishi as Director General for the successor of Tadaharu Goto, and Sachiko Nakagawa as Managing Director for the successor of Akihiko Matsubara, effective April 1st, 2020.
JPMA appreciate and remember Tadaharu Goto and Akihiko Matsubara for their services provided to innovative pharmaceutical industry in Japan for long time.Junichi Siraishi has impressive work experience at Ministry of Health, Labor and Welfare and Ministry of Environment before he served as an adviser at the Sumitomo Mitsui Banking Corporation since 2014. On the other hand, Sachiko Nakagawa has been served to a JPMA member company through her carrier as an educator and an executive member and her latest position at the company was head of China Operations Management Office since 2017.
We JPMA are very excited to advance our organization with experienced and talented new leaders from next month. Please welcome our new leaders, and let’s create far strong collaborative atmosphere with APAC members together.
-
The 9th APAC Conference Post-Ups:Cancellation Notice
We are very sorry to inform you that the 9th Asia Partnership Conference of Pharmaceutical Associations (the 9th APAC) planned to be held on April 7, 2020 at Tokyo have been cancelled, due to the outbreak of COVID-19.
We hope that the current worldwide confusion and disaster to be solved as soon as possible without any serious outcomes happen, and we wish we can announce you holding the 10th APAC conference in the definite future. -
The 9th APAC Conference Post-Ups:Topics for Sessions
The next APAC meeting will take place on April 7 in Tokyo. It will offer a wide range of topics to facilitate interactions between regulatory authorities, academia and pharmaceutical industries as follows:
-
1)Regulations and Approvals
-
Reliance Pathway
-
-
2)VBH
-
Approach Sustainable Healthcare System
-
-
3)Access to Innovative Medicines
-
BCS (Biopharmaceutics Classification System)-based Biowaiver (ICH M9)
-
-
4)Drug Discovery Alliances
-
Progress of DA activities to promote cross-border open innovation in Asia
-
-
-
The 8th APAC Meeting: Post-Meeting Follow-Up (Presentation Materials Available)
Thank you for waiting so long. All available presentation materials used at the 8th APAC conference was uploaded on this homepage today. Please visit the site ACHIEVEMENTS or click
In addition, Chairman of JPMA also mentioned about the achievement of the 8th APAC in his message column briefly.
-
The 8th APAC Conference Post-Ups: Topics for Sessions
The conference will take up the issue of Value-based Healthcare at the morning. There will be a keynote lecture as well as panel discussion with insightful panelists.
Regular topics will follow after the panel discussion. Those are;-
1)Access to Innovative Medicines
-
Change Control
-
-
2)Regulations and Approvals
-
Good Registration Management
-
Reliance Pathway
-
-
3)Drug Discovery Alliances
-
Revitalization of drug discovery using natural products by open innovation in Asia
-
The conference will be facilitated with participants of regulatory authorities, academia and pharmaceutical industries in Asia. We believe you would satisfy the session and feel it was very much informative.
-
-
APAC Natural Products Drug Discovery Consortium
The signing ceremony for APAC Natural Products Drug Discovery Consortium was held by the ASAP* representatives of Japan (JPMA), Thailand (TCELS), and Taiwan (BPIPO) at the JPMA on Oct.12.
-
*ASAP: Aggregate Services Access point

-
-
The 7th APAC Meeting: Post-Meeting Follow-Up (Presentation Materials Available)
With all of attendees’ support, the 7th APAC conference was successfully held on April 10th, 2018 at Keidanren-Kaikan in Tokyo with more than 350 participants including regulators, academia and industries. We are pleased to announce you that we have uploaded all the presentation materials on this homepage by today. We invite you to visit the ACHIEVEMENTS.
The new president message from JPMA
Please also visit ABOUT US – Welcome message. You will find a message from Mr. George Nakayama, the new president of JPMA elected on May 24th, 2018. -
The 7th APAC Meeting Post-Ups: Topics for Sessions
The next APAC meeting will take place on April 10 in Tokyo. It will offer a wide range of topics to facilitate interactions between regulatory authorities, academia and pharmaceutical industries as follows:
-
1)Regulations and Approvals
-
Good Registration Management
-
Conditional Early Approval Systems
-
-
2)Drug Discovery Alliances
-
How to establish drug discovery ecosystem in Asia
-
-
3)Access To Innovative Medicines
-
Site Master File
-
Change Control
-
We hope that your stay in Tokyo will be informative, fruitful and, last but not least, enjoyable.
-
-
APAC DA-EWG Events at BioJapan were held on October 10-12, 2017
BioJapan 2017 was held from October 11 (Wed.) to October 13 (Fri.) and APAC’s DA-EWG carried out the following four activities:
-
ⅰ)APAC DA-EWG Research Center Site Visit (Day 0 (Oct.10))
-
ⅱ)APAC Business Development Partnering Conference (Day 1 (Oct.11))
-
ⅲ)APAC Bio Japan Session "Revisit on the potential of natural compounds in drug discovery" (Day 2 (Oct.12))
-
ⅳ)APAC DA-EWG All-Member Meeting (Day 2 (Oct.12))
-
-
The 7th APAC Meeting Post-Ups: Date and Venue
Save the date to join us at the 7th APAC Meeting in Tokyo, April 10, 2018.
The Meeting will be held at Keidanren Kaikan located in 1-3-2, Otemachi, Chiyoda-ku, Tokyo. -
APAC welcomes a new member
APAC is delighted to welcome China Pharmaceutical Innovation & Research Development Association (PhIRDA) as a new member.
Click Here For More Info about PhIRDA!
-
APAC DA-EWG: HP has been updated
The information of APAC 6th concept paper, Pillar4 and Pillar 5 has been uploaded.
-
The 6th APAC Meeting: Post-Meeting Follow-Up (Presentation Materials Available)
As a reminder, we have uploaded all the presentation materials. We invite you to visit the ACHIEVEMENTS.
-
The 6th APAC Meeting: Thank You for the Conference Participants
Thank you very much for everyone who participated in the 6th APAC in Tokyo. The weather, cherry blossoms and session presentations/discussions made the conference successful. Over 320 attendees were exposed to informative and worthwhile topics related to Access To Innovative Medicines, Drug Discovery Alliances, Regulations & Approvals and Keynote Lecture by outstanding speakers. We will continue our efforts to accomplish our mission.
-
The 6th APAC Meeting: Meeting Program and Venue
Dear Sirs,
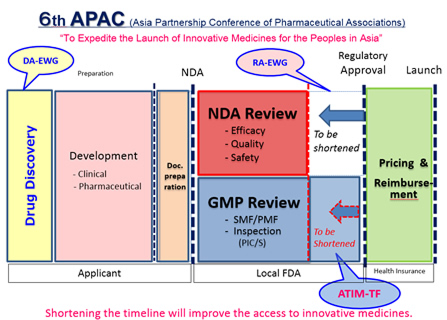
We are pleased to announce that the 6th APAC meeting will be held in Tokyo Conference Center Shinagawa on April 5, 2017. APAC is an industry-government cooperative initiative led by R&D-based pharmaceutical associations in Asia, aiming to fulfill its mission, i.e., “To expedite the launch of innovative medicines for the peoples in Asia”. In consideration of the recent movements of Asian governments’ healthcare enhancement, especially in GMP/Quality area, we will have a session on GMP in the “Access To Innovative Medicines (ATIM)” session.In addition to the ATIM session, we will have “Drug Discovery Alliances (DA)” session to be discussed about drug discovery cross-border open innovation using natural resources and “Regulations & Approvals (RA)” of which theme is how to extend GRM (Good Registration Management) guideline in Asia. Please see the attached program.
Tokyo Conference Center Shinagawa
-
The 6th APAC Meeting: General Information
When is the 6th APAC Meeting?
April 5, 2017When does the last sessions end?
Sessions will run through the evening of April 5What is going to be held on Day 2?
APAC Convention (Closed, Members Only) -
The 9th Asia regulatory Conference (ARC): Register Now!
The 9th ARC will be held in Tokyo, 6-7 April, 2017. The online registration system is now open.
For more information, please follow this link.
-
The 6th APAC News
The 6th APAC will take place on April 5 and 6, 2017 in Tokyo. The conference will offer a wide range of topics including:
-
ATIM Session
-
Promoting Efficacy in GMP Review
-
-
DA Session
-
Dawning Era in Drug Discovery with Natural Resources in Asia
-
-
RA Session
-
Toward Efficient & High-Quality Registration Process for Innovative Medicines
-
Save the date to join us in Tokyo!
Details coming soon.
-
-
APAC RA-EWG collaborated with APEC LSIF RHSC for the Regulatory Science Center of Excellence Pilot Workshop on Good Registration Management (GRM) held in Taipei on November 15-17.
APEC Good Registration Management Regulatory Science CoE (Center of Excellence) pilot workshop was held in Taipei on November 15-17, 2016. APAC RA-EWG participated in this kick-off workshop as one of the co-organizers and supported the Applicant-specific session on the Good Submission Practices.
-
APAC DA-EWG Events at BioJapan were held on October 11-14 successfully
BioJapan 2016 was held from October 12 (Wed.) to October 14 (Fri.) and APAC’s DA-EWG carried out the following four activities:
-
ⅰ)APAC DA-EWG Research Center Site Visit (Day 0 (Oct.11))
-
ⅱ)APAC Business Development Partnering Conference (Day 1 (Oct.12))
-
ⅲ)APAC Bio Japan Session "Modern Natural Products Drug Discovery in Asia" (Day 2 (Oct.13
-
ⅳ)APAC DA-EWG All-Member Meeting (Day 3 (Oct.14))
-
-
JPMA’s New President Mr. Hatanaka posted Welcome Message
-
The 5th APAC materials have been listed up in the Achievements
The outline and presentation materials of The 5th APAC meeting have been uploaded in the achievements.
-
The 5th APAC was held on April 7-8 successfully
The Fifth APAC (Asia Partnership Conference of Pharmaceutical Associations) was held on 7th and 8th April with the mission of “To Expedite the Launch of Innovative Medicines for the Peoples in Asia” under the theme of “APAC’s continues challenge to create and improve access to innovative medicines”.
-
The 5th APAC meeting at Tokyo on April 7-8, 2016
Dear Sir / Madam,
On behalf of APAC (Asia Partnership Conference of Pharmaceutical Association), we are pleased to inform you that the Fifth APAC meeting will be held in Tokyo on April 7-8, 2016 under the semi-closed basis.
APAC is an industry-driven initiative led by R&D-based pharmaceutical associations from Asian countries, aiming to fulfill the mission of “to expedite the launch of innovative medicines for the peoples in Asia”. And, the theme of the 5th meeting is “APAC’s further challenges for the creation of innovative medicines and improving access to innovative medicines”.
In consideration of the recent movements of Asian governments’ healthcare reforms, we will have an Access To Innovative Medicines (ATIM) session at the next meeting inviting as many high ranking officers, and to discuss “what APAC can contribute toward establishing public private partnerships that will enable patients to access the innovative medicines much faster”, in addition to two expert working group session of “Regulations & Approvals” and “Drug Discovery Alliances”. Just for your reference, please see the attached program draft.
We will feedback the outcome of the event on this web-site soon after completion of the meeting.